New stem cell treatment could repair any tissue in the body using fat cells
Australian scientists have discovered an ingenious way to reprogram adult bone or fat cells to form stem cells, creating the potential to regenerate any damaged tissue in the body.
Inspired by the limb-replacing ability of salamanders, the team developed a technique that "gives adult cells the ability to lose their adult characteristics," according to Science Alert. They can then "multiply and regenerate multiple cell types - what is known as multipotency. That means the new stem cells can hypothetically repair any injury in the body, from severed spinal cords to joint and muscle degeneration. And it’s a pretty big deal, because there are currently no adult stem cells that naturally regenerate multiple tissue types."
"This technique is a significant advance on many of the current unproven stem cell therapies, which have shown little or no objective evidence they contribute directly to new tissue formation," said lead researcher John Pimanda from the University of New South Wales, Faculty of Medicine (UNSW Medicine). "We are currently assessing whether adult human fat cells reprogrammed into [induced multipotent stem cells (iMS cells)] can safely repair damaged tissue in mice, with human trials expected to begin in late 2017."
Currently, stem cell therapy is limited. Most use embryonic stem cells, which are taken from developing embryos. The problem with these is that they can form tumours and are not able to be transplanted directly to regenerate adult cells.
Another current option is tissue-specific adult cells, which can only be used in cells in their region of the body - for example, lung stem cells can only be used in lung tissue, which causes many restrictions.
"Embryonic stem cells cannot be used to treat damaged tissues because of their tumour forming capacity," said one of the researchers, Vashe Chandrakanthan. "The other problem when generating stem cells is the requirement to use viruses to transform cells into stem cells, which is clinically unacceptable.""We believe we’ve overcome these issues with this new technique."
In order to make these new type of stem cells, the researchers collected adult human bone and fat cells and treated them with two compounds: 5-Azacytidine (AZA); and platelet-derived growth factor-AB (PDGF-AB) for two days.
"This kick-started the process of dedifferentiation – which basically means it started to revert them to a multipotent stem cell state. The cells were then kept in PDGF-AB for a few weeks while they slowly changed into stem cells, eventually becoming tissue-regenerative iMS cells – which basically means they can repair any type of tissue in the body."
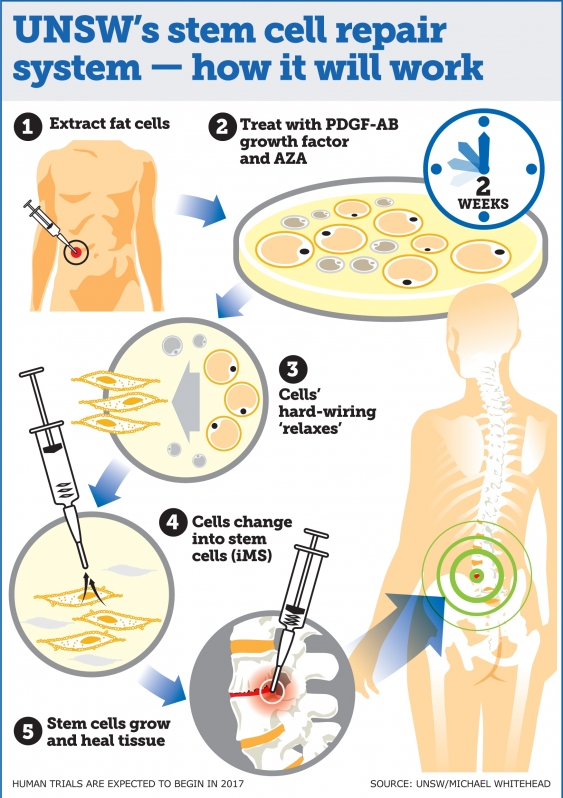
"This technique is ground-breaking because iMS cells regenerate multiple tissue types," said Pimanda. "We have taken bone and fat cells, switched off their memory and converted them into stem cells so they can repair different cell types once they are put back inside the body."
The researchers are still looking into how the cells act at sites of transplantation. If all goes well, human trials are expected for late 2017. Early trials will focus on whether the iMS cells can heal bone, joint, and muscle tissue.
This research has been published in the Proceedings of the National Academy of Sciences.